Topical silicone solutions for ostomy care
Formulate topical barrier film and adhesive removal products for ostomy patients
Ostomy care products that are gentle, effective, pleasant-feeling and easy to use encourage patients to take better care of the skin around their stoma. When properly treated, the skin stays healthier and the patient experiences less discomfort, making life with an ostomy a more positive experience.
Silicone technologies, formulation expertise and regulatory support from DuPont can help you accelerate the development and approval of ostomy care topical solutions that meet patients’ needs for comfort, convenience and protection.
Begin with ready-to-use silicone blends from DuPont™ Liveo™
DuPont has used its expertise in formulating silicone-based materials for topical applications to bring you a series of pre-blended topical silicone solutions suitable for use in ostomy care products:
• Liveo™ MG-2107 Silicone Blend and Liveo™ MG-2110 Silicone Blend for skin barrier products
• Liveo™ MG-2001 Silicone Blend for adhesive removal products
These proven, ready-to-use blends can help you reduce formulating steps and mitigate volatile-handling challenges encountered in manufacturing. Additionally,
their quick-drying, non-tacky, non-occlusive nature combined with their pleasurable silicone feel can help you create products that increase patient comfort and compliance.
Solutions from a globally recognized leader in silicone-based healthcare material innovation
- Proven silicone technology for medicalproduct development
- Ready-to-use silicone blends to accelerate your formulation development
- Breathable film-formers with good washoff resistance for skin barrier properties
- An efficient, non-stinging cleaning agent for adhesive removal
- A level of efficacy that contributes to patient compliance
- Formulation, regulatory and product development expertise and support


Topical silicone solutions for ostomy care applications
Clear, colorless and low-viscosity, these 100% silicone blends can be used as bases for topical sprays or wipes. Due to their high volatility, they also are useful as carrier solvents for topical sprays.
Silicone blends for skin barrier products
INCI Name: Disiloxane (and) Trisiloxane (and) Trimethylsiloxysilicate (Note: INCI Name is the same for both products.).
Performance testing summary
The performance of Liveo™ MG-2107 Silicone Blend and Liveo™ MG-2110 Silicone Blend was compared to the performance of a commercial benchmark product1.
Film substantivity – The Liveo™ silicone blends and the benchmark all exhibited good substantivity over time (Figure 1) and good wash resistance (Figure 2).
Tackiness – Liveo™ MG-2107 Silicone Blend exhibited lower tackiness than the benchmark (Figure 3).
Drying time – Both of the Liveo™ silicone blends exhibited comparable drying time to the benchmark (Figure 4).
Perceived film presence – Each of the Liveo™ silicone blends exhibited a higher film presence than the benchmark.
Non-occlusivity/breathability – The Liveo™ silicone blends and the benchmark demonstrated non-occlusive properties, which allow the skin to breathe.
Illustrative test results
Silicone blend for adhesive removal products
INCI Name: Disiloxane (and) Trisiloxane (and) Dimethicone
Performance testing summary
The performance of Liveo™ MG-2001 Silicone Blend was compared to the performance of a European commercial benchmark product² and a U.S. commercial benchmark product3.
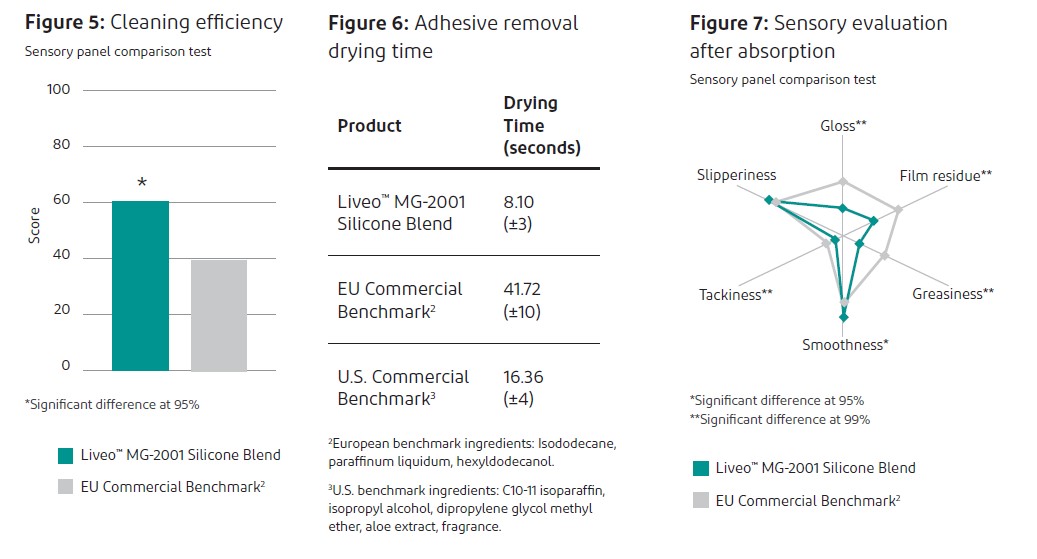
Cleaning efficiency – Liveo™ MG-2001 Silicone Blend performed better than the European commercial benchmark (Figure 5).
Drying time – Liveo™ MG-2001 Silicone Blend dried significantly faster than either the European commercial benchmark or the U.S. benchmark (Figure 6).
Sensory profile – Compared to the European benchmark and the U.S. benchmark, Liveo™ MG-2001 Silicone Blend exhibited less film residue, less greasiness and less tackiness. (See European commercial benchmark comparison, Figure 7.)
Illustrative test results
Make DuPont your innovation leader
With more than 70 years of healthcare market experience in the design of silicone-based systems, you can rely on
DuPont for:
- A reliable global supply of reputable, cost-effective silicone-based topical ingredients manufactured specifically for healthcare applications
- Proven aesthetics and functional film characteristics, such as breathability and substantivity
- Test data to support specific claims
- Formulation, regulatory and product development expertise and support
- A regulatory information package aligned with the requirements of the consumer healthcare market
Would you like to learn more, or request a sample?

To learn more about DuPont’s healthcare solutions visit:
www.dupont.com/healthcare.html
For country-level information, visit:
www.dupont.com/corporate-functions/our-company/global-locations.html

CAUTION: DO NOT USE DUPONT MATERIALS IN MEDICAL APPLICATIONS INVOLVING PERMANENT IMPLANTATION IN THE HUMAN BODY OR
PERMANENT CONTACT WITH INTERNAL BODILY FLUIDS OR TISSUES. DO NOT USE DUPONT MATERIALS IN MEDICAL APPLICATIONS INVOLVING
BRIEF OR TEMPORARY IMPLANTATION IN THE HUMAN BODY OR PERMANENT CONTACT WITH INTERNAL BODILY FLUIDS OR TISSUES UNLESS
THE MATERIAL HAS BEEN PROVIDED DIRECTLY BY DUPONT UNDER A CONTRACT THAT EXPRESSLY ACKNOWLEDGES THE CONTEMPLATED USE.
The information, suggestions and data contained herein are intended only as an informational guide to assist you in making preliminary selections of materials
and are not intended to be all-inclusive or final. Because DuPont cannot anticipate or control the many different conditions under which this information, data,
suggestions or materials may be used, DuPont does not guarantee the applicability or the accuracy of this information or the suitability of the information, data,
suggestions, or materials in any given situation. The information, data, or suggestions are not intended to substitute for any testing you may need to conduct to
determine for yourself the suitability of a particular material for a particular purpose. DuPont makes no guarantee of results and assumes no obligation or liability
whatsoever in connection with this information. Such information, data or suggestions are to be used and relied upon at user’s own discretion and risk. DuPont
makes no warranties, express or implied, and disclaims any and all direct and indirect liability for damages or losses resulting from or relating to the use of any
information, suggestion, data, or materials described herein. Statements concerning the use of the products or formulations described herein are not to be
construed as recommending the infringement of any patent, copyright, designs or other intellectual property and no liability for infringement arising out of such use
is assumed by DuPont. None of this information is to be considered as a license to operate under, or recommendation to infringe, any patents.
DuPont reserves the right not to sell Special Control and Premium Control products for selected applications.
Although these products are tested against certain USP Class VI and ISO 10993 standards, DuPont makes no representation or warranty of suitability of its
products for particular healthcare or medical applications or any other representations or warranties based on such testing.
The information set forth herein is furnished free of charge and is based on technical data that DuPont believes to be reliable. It is intended for use by persons having
technical skill at their own discretion and risk. DuPont makes no warranties, express or implied, and assumes no liability in connection with any use of this information.
DuPont™, the DuPont Oval Logo, and all products, unless otherwise noted, denoted with TM, SM or ® are trademarks, service marks or registered trademarks
of affiliates of DuPont de Nemours, Inc.
© 2020 DuPont de Nemours, Inc. All rights reserved.